Abstract
BACKGROUND Allogeneic hematopoietic stem cell transplants (alloHSCT) remain the only curative treatment for many advanced hematologic malignancies; however, despite improvements in donor matching and supportive care, these transplants are associated with significant toxicity and reduced quality of life. Strategies to maintain or enhance the graft-vs-tumor and graft-vs-infection effects while eliminating graft-vs-host disease (GVHD) have long been a key goal in the field.
Orca-T is a high-precision, immunotherapy consisting of stem and immune cells, derived from allogeneic donors, that leverages highly purified, polyclonal donor regulatory T cells to control alloreactive immune responses. Orca-T has demonstrated a favorable safety profile and promising control of both relapse and GVHD.
METHODS As of 15 July 2022, 180 patients have received Orca-T. Of those patients, 127 have ≥180 days of follow-up and a diagnosis of acute leukemia in CR [AML (n=62), ALL (n=42), mixed phenotype acute leukemia(n=4), CML with prior blast crisis (n=4)], or high risk MDS (n=15). Patients received Orca-T as part of a single-center Phase 2 study (n=32, NCT01660607) or a multicenter Phase 1b study (n=95, NCT04013685). Median follow-up was 387 days (range 41-2062); 72 and 36 patients have > 1 year and >18 months of follow-up, respectively. Patients were aged 19-69 (median 48) and 57% male. Donors were HLA-matched related (n=66) or unrelated (n=61). Patients received investigator's choice of myeloablative conditioning regimens (busulfan-based, n=98; TBI-based, n=29) prior to Orca-T, followed by single-agent GVHD prophylaxis with either tacrolimus (n=124) or sirolimus (n=3). For comparison purposes, an independent CIBMTR-based cohort was identified which consisted of patients with AML, ALL, or MDS who received myeloablative alloHSCT with a PBSC source between 2016-2018 followed by tac/methotrexate PPX. Matching for relapse risk was based on minimal residual disease (MRD) status in acute leukemia patients; development of an additional risk matched CIBMTR comparator based on DRI score and MRD status is ongoing.
RESULTS Orca-T was successfully manufactured in a single, centralized GMP manufacturing facility, distributed throughout the U.S., and infused for all patients enrolled.
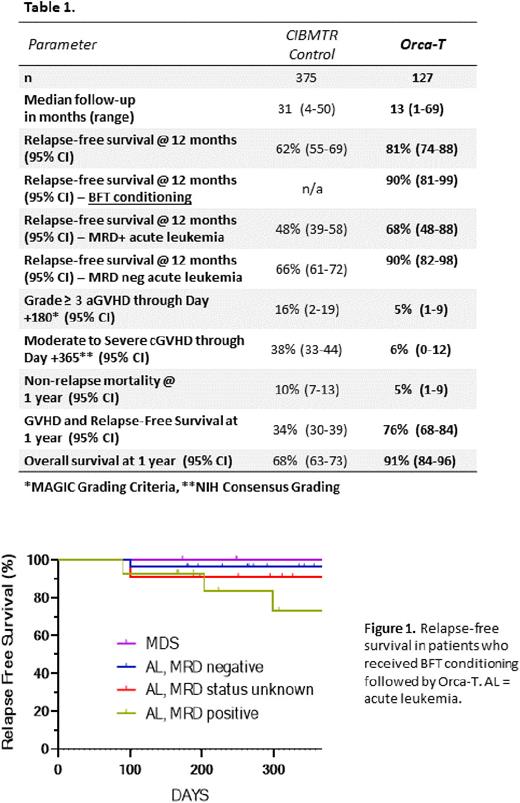
The relapse-free survival was 81% at both 1 year and 18 months in Orca-T recipients. MRD status was determined for 77 patients with acute leukemia by multicolor flow cytometry; of these patients, 31% were MRD+ when they received Orca-T. Amongst MRD- patients (n=53), RFS with Orca-T was 90% at both 1 year as compared to 66% in the CIBMTR cohort (n=324 MRD- patients). Amongst MRD+ patients (n=24), RFS was 68% at one year with Orca-T as compared to 48% in the comparator cohort (n=104).
Relapse prevention with Orca-T appeared to be enhanced further with a conditioning regimen consisting of busulfan, fludarabine, and thiotepa ("BFT", n=56 patients, median f/u 342 days); RFS was 90% at 12 months in this group. This included patients with MDS (n=6, 100% RFS at 1 yr), MRD+ acute leukemia (n=14, RFS 73% at 1 yr), MRD- acute leukemia (n=26, RFS 96% at 1 year), and acute leukemia with unknown MRD status (n=11, 91% RFS at 1 yr) (Figure 1).
As with relapse, severe infections were low following Orca-T with 11% of patients developing Grade 3 infections per the BMT-CTN grading scale.
Median times to neutrophil and platelet engraftment were rapid with Orca-T at 13 and 16 days, respectively; graft failure was rare at 1.6%. Grade ≥ 3 aGVHD and mod/severe cGVHD rates were low with Orca-T at 5% and 6%, respectively, through 1 year post-transplant. Non-relapse mortality was low at 5% at 1 year; NRM with BFT was 0%. Overall, Orca-T shows GRFS of 76% and 69% at 1 year and 18 months post-transplant, respectively; OS was 91% and 86% at these time points post-transplant. No formal statistical comparison to the CIBMTR cohort was performed (Table 1).
CONCLUSIONS At more than 1 year of median follow-up, outcomes with Orca-T, a high-precision immunotherapy, demonstrate robust graft-vs-leukemia and graft-vs-infection effects while markedly reducing GVHD and NRM despite MAC. These outcomes were accomplished with consistent and reliable cell manufacturing and distribution of Orca-T at a national scale. A multi-center randomized controlled phase 3 trial comparing Orca-T to SOC, utilizing BFT or TBI-based conditioning, is currently enrolling across the US (NCT05316701).
Disclosures
Oliai:Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding; Orca Bio: Research Funding; Arog: Research Funding; Seagen: Research Funding. Hoeg:Orca Bio: Research Funding. Pavlova:Orca Bio: Current Employment, Current holder of stock options in a privately-held company, Research Funding. Gandhi:Orca Bio: Research Funding; Gamida: Honoraria; CareDx: Consultancy. Mehta:Orca Bio: Research Funding; Syndax: Research Funding. Srour:Orca Bio: Research Funding. Waller:Orca Bio: Research Funding; Verastem Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lowsky:Orca Bio: Research Funding. Patel:Medexus Pharma: Honoraria; CareDx: Honoraria; Orca Bio: Research Funding; Kite: Honoraria. Dholaria:Molecular Templates: Research Funding; Takeda: Research Funding; Wugen: Research Funding; MJH Biosciences: Honoraria; BEAM Therapeutics: Consultancy; Orca Bio: Research Funding; Poseida: Research Funding; Pfizer: Research Funding; Arivan: Consultancy; Janssen: Research Funding; Gamida Cell: Consultancy; Vanderbilt University Medical Center: Current Employment; Angiocrine: Research Funding; BMS: Research Funding; MEI Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy. Pantin:NKARTA: Consultancy; Omeros Corporation: Consultancy, Speakers Bureau; Cardinal Health: Honoraria; Orca Bio: Research Funding. Salhotra:Kadmon: Other: Advisory board meeting ; Orca Bio: Research Funding; BMS: Research Funding. McGuirk:CRISPR Therapeutics: Consultancy; Sana: Honoraria; Orca Bio: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Nextar: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; In8bio, Inc.: Other: IIT Clinical Trial. Fernhoff:Orca Bio: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. McClellan:Orca Bio: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Abedi:Celgene: Consultancy, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Orca Bio: Research Funding; AbbVie: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau; CytoDyn: Current equity holder in publicly-traded company. Negrin:CoImmune: Current equity holder in private company, Current holder of stock options in a privately-held company; University of Pennsylvania: Other: DSMB or Advisory Board; Novartis: Consultancy; UptoDate: Honoraria; Amgen: Consultancy; Kuur: Consultancy; Garuda: Consultancy; BioEclipse Therapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company; Magenta: Consultancy, Current equity holder in publicly-traded company. Meyer:GigaGen: Other: Co-founder, scientific advisor; indee labs: Membership on an entity's Board of Directors or advisory committees; Triursus Therapeutics: Other: Co-founder, scientific advisor; Orca Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal